Medical Device Contract Manufacturing Services for Box Build or Subassemblies
Save Costs, and Outsource your Manufacturing Needs with MFG One
Full-Service Contract Manufacturer for Medical Device
Get Support at Any Stage of the Development Process
From the drawing board to key stages of product development, get the support you need at an affordable price with our open-book pricing. Free up your floor space and reduce overhead costs by outsourcing to MFG One. With extensive expertise across medical and pharmaceutical devices, we’re here to bring your product vision to market with precision and quality.
Check out our services below to see how we can support you on your medical device manufacturing journey.
Our Manufacturing Support Services
Discover how we can help your business grow. Select a service tab to find the perfect solution for your needs.
Our concept and design services take your initial ideas and develop them into robust, manufacturable products ready for the market.
Engineering and Design Services
- 2D-3D Conversion
- Biomedical Engineering Services
- Cost Analysis
- Design & Development
- Electrical & Software Engineering
- Electro-Mechanical Engineering
- Engineering Support
- Failure Analysis & Reliability
- FEA (Finite Element Analysis)
- Human Factors Engineering
- Injection Mold Design
- Mechanical Design Services
- New Product Introduction (NPI)
- Packaging Engineering
- Plastics Engineering
- Product Development
- Prototype Engineering
- Quality Engineering
- Reverse Engineering
- Simulation and Testing
- Software Development Services
- Solidworks
- Sustaining Engineering
- Technical Documentation
- Test Development
- Value Engineering
Design with manufacturing in mind, providing a smooth transition from concept to production while maximizing efficiency and quality.
Design for manufacturability Services
- Optimizing Design for Cost-Effective Production
- DFX (Design for Excellence)
- DFT (Design for Test)
- DFMA (Design for Manufacturing and Assembly)
- VAVE Services
- Lean Manufacturing
Transform ideas into functional designs with our expert engineering and development services, optimized for real-world applications.
Engineering Services
- Product Development
- Electro-mechanical Engineering
- Tool Engineering
- Prototyping Engineering
- Manufacturing Process Engineering
- Test Engineering
- Firmware and Software Development
- Packaging Engineering
- Product Engineering
- Metal Engineering
- Quality Engineering
Engineering Capabilities
- 2D-3D Conversion
- Design and Development
- Failure Analysis & Reliability
- FEA (Finite Element Analysis)
- Injection Mold Design
- NPI Engineering Plastics
- Engineering Product Development
- Reverse Engineering
- Simulation and Testing
- Mechanical Design Services
- Value Engineering
- Solidworks
- Sustaining Engineering
- Technical Documentation
- Test Development
- Software Development Services
Our manufacturing and assembly services deliver your product concepts with accuracy, high quality, and scalability.
Core Capabilities
- Turn-Key Complete Box-Build Assembly
- Printed Circuit Board Assembly
- Plastic Injection Molding
- Mold Tooling
- Metal Fabrication
Secondary Operations
- Silk Screening & Pad Printing
- EMI Shielding
Thorough testing and validation services to help your products meet top performance and compliance standards, with FDA support backed by our legal team.
Testing Services
- Vibration
- Mechanical Shock
- Drop
- Mechanical Life
- Force, Displacement, and Torque
- Environmental
- Measurements and Visual Inspection
FDA Support
- FDA Approval Guidance
- Legal Team Support
Validation Services
- Verification/Validation of Final Unit
- Verification/Validation of Software
- Packaging Design/Validation
- Risk Assessment
- Design Failure Mode and Effects Analysis (DFMEA)
- Process Failure Mode and Effects Analysis (PFMEA)
Specialized Validation & Compliance Support
- GMP, cGMP, GLP, GAMP
- IQ/OQ/PQ
- Calibration Management
- CFR 21, Part 11 Electronic Signatures, Electronic Records Consulting
- Computer system Validation
- Design Specification
- Equipment Validation
- Facility Qualification
- FAT & Commissioning
- Final Validation Reports
- Functional Specification
- Process Validation
- Requirements Analysis
- Risk Assessment
- Site acceptance Tests (SAT)
- Software Requirements Specifications (URS)
- Standard Operating Procedures SOPs
- System Validation
- Traceability Matrices
- User Requirements Specifications (URS)
- Validation Gap Analysis
- Validation Master Plan Development
- Validation Program Management
- Vendor Qualification
Our 100,000-square-foot, temperature-controlled facility provides the ideal environment for reliable logistics and fulfillment solutions.
Supply Chain Management
- Strategic Commodity Procurement & Supplier Management
- Cost Reduction Throughout the Product Life Cycle
- End-of-Life Materials Management
- EDI/Bonding Programs
- Review of Suppliers Quality, Delivery, Pricing
- Web-based Access to Parts Inventory
- Database Inventory Control Systems International Sourcing
- Component Traceability
- Precise Labeling
- Professional Packaging
Third Party Logistic (3PL) Services
- Assembly
- Distribution Services
- Transportation Services
- Regulatory Services
- Inventory Management
- Returns Management
Inventory Control Systems
- Stocking Programs with Suppliers
- KANBAN Inventory Program
- Consignment Inventory Program
Extend the life and reliability of your products with MFG One’s aftermarket support tailored to meet ongoing customer needs.
Aftermarket Support Services
- Management of Product Lifecycle
- Aftermarket and Remanufacturing Services for OEMs
- Return Management
- Inventory Management
- Warranty
- Screening and Triage
- 3PL Fulfillment Services
- Repair Services
- Failure Analysis
- Refurbishment & Remanufacturing Services
Additional Value Added Services
- Engineering Support DFM
- Access 24/7 Manufacturing
- Customer Service Centers
- Program Management
- IP Protection
- Global Logistics
- Project Management
- Full Life Cycle Management
- VA/VE
- Refurbishment & Remanufacturing Services

Turn-Key Complete Box-Build Process
We handle every part of the manufacturing process, start to finish. Whether you need our expertise for a single step or support throughout the entire process, we’re here to help you build quality products that meet your exact standards.
DFM: Design For Manufacturability
- Engineering Samples
- Executes Pilot Runs
- Transition Your Product to Full Production
- Meet Your Schedules and Overall Demands
Once your product is designed and ready for production:
- We make sure all issues from the engineering samples and pilot runs have been fully addressed.
Ongoing Production
The VA/VE roadmap starts with your product’s evolving needs, to meet changing marketplace requirements.
Rigorous final checks for optimal market performance.
Continuous Improvement
We work with your 12-month rolling product forecast:
- VA/VE Teams
- Supply Chain Planning
- Supply and Cost Competitiveness
Cost Savings
- Identify Alternative Materials
- Refine Production Methods
- Drive Out Waste
- Establish Lean Manufacturing Processes
- Recommend Enhancements
Get Consistent Support from Our US-Based Contract Manufacturing Faciliy
As a U.S. contract manufacturer, we’re here to support customers worldwide. From full-scale production to shipping, partnering with us means fewer delays and no costly tariffs.
Expertise Across Wide Range of Medical & Pharmaceutical Devices
Our expertise across diverse medical and pharmaceutical device ensures your product is engineered and manufactured to the highest quality standards. Our experience encompasses the creation of medical devices, such as the following:
- Diagnostics Equipment
- Electromechanical Device
- Laboratory Equipment
- Life Science / Laboratory Products
- Patient Care and Monitoring
- Personal Use of Medical Devices
- Point of use Diagnostics
- Healthcare
- Patient Monitoring
- Drug Delivery
- Med Tech
- Anesthesia & Respiratory
- Surgical Systems
- Much More
Medical Devices We Manufactured
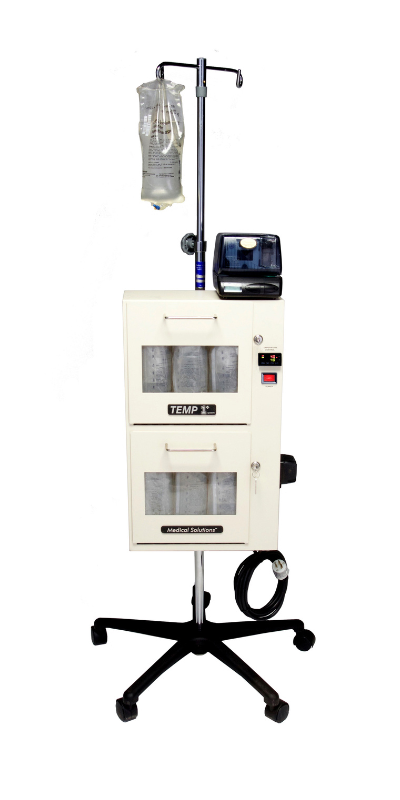
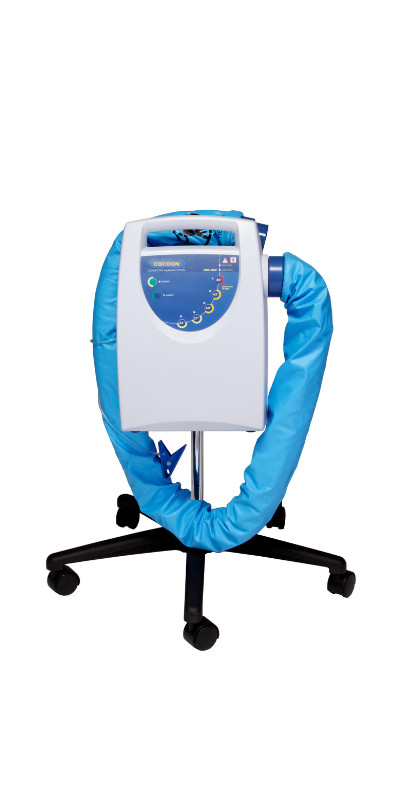




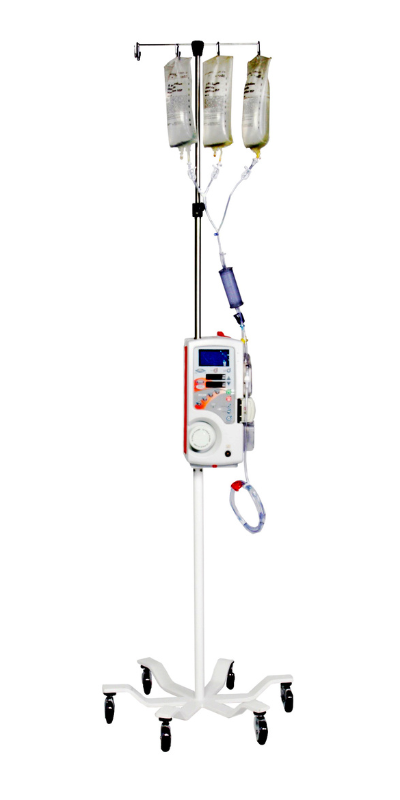
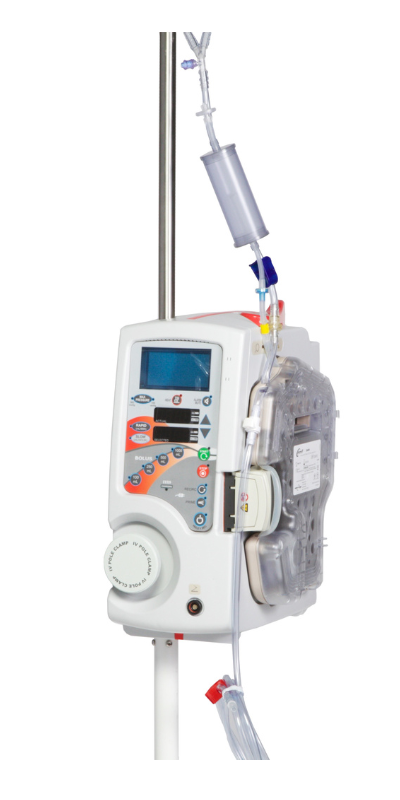

Why Outsource With MFG One?
- Open Book Costing
Full transparency into material, labor, and overhead costs for better decision-making.
- Reduce Operating Costs
Strategies to streamline processes and lower overall expenses.
- PCBAs, Plastics, Metals, Full Assembly
Diverse manufacturing services from PCBAs to full assembly, all under one roof.
- Program Manager Led Cross-Functional Teams
Dedicated program managers ensure projects stay on track, on budget, and aligned with your goals.
- Centralized US-based in Chantilly, VA
Our Chantilly, VA facility supports efficient logistics, fast communication, and quality control for US clients.
