Table of Contents
3 Key Stages in Medical Device Product Development
Successfully bringing a medical device to market requires following a structured product development process. Each phase is vital to transforming your concept into a reliable, compliant product ready for market. Working with a manufacturer that offers design and engineering services helps smooth out transitions, saving time and reducing potential risks.
Here are the key stages of medical device product development:
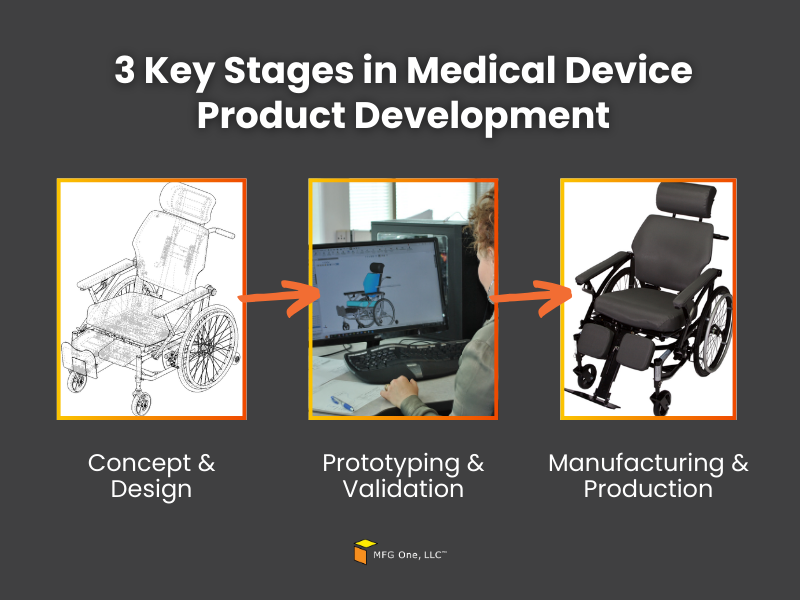
- Concept and Design: This stage focuses on refining your product idea and translating it into a functional design. Partnering with experts in product engineering maintains that your product is optimized for both performance and manufacturability from the very start.
- Prototyping and Validation: Once the design is finalized and the product is engineered, the next step is prototyping. During this stage, the product is tested to verify its functionality. Along with process validation, this phase makes sure the product meets performance expectations and adheres to US regulations, which is especially important for FDA registered medical device manufacturers.
- Manufacturing and Production: After the validation stage, your product moves into full-scale production. This is where working with a trusted contract manufacturing services provider becomes essential to keeping your project on track, maintaining quality, and maintaining regulatory compliance.
How to Improve Time-to-Market
Speed is everything when it comes to bringing a medical device to the US market. The faster you can move through key milestones, the sooner your product can make an impact. One way to accelerate your timeline is by partnering with a company that offers comprehensive design and engineering solutions. These services focus on optimizing your product from the start, keeping critical validation requirements in mind to streamline the entire process.
Here’s how design services can help:
- Validation Built-In: When a manufacturer keeps process validation at the forefront, it minimizes delays. By integrating validation into each phase, from concept to production, you avoid last-minute compliance issues that can slow things down.
- Optimized Prototyping: Speeding through the prototyping stage without cutting corners is key to hitting your launch goals. Working with a team that understands medical device design and manufacturing will help you move quickly through this stage while keeping quality and compliance intact.
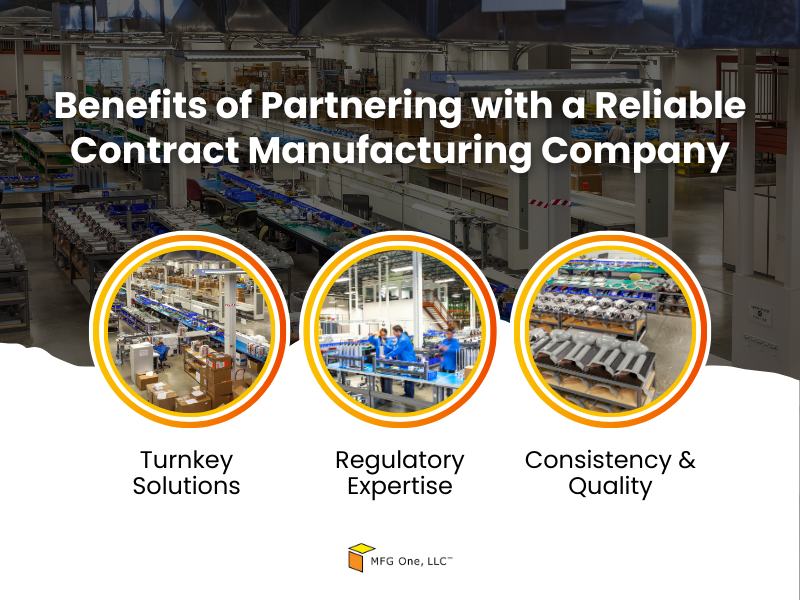
Benefits of Partnering with a Reliable Contract Manufacturing Company
Choosing the right contract manufacturing services provider can make all the difference when it comes to getting your medical device to market. A trusted partner offers not only technical expertise but also a comprehensive approach to product development. With a trusted contract manufacturer, you gain access to turnkey solutions that streamline every step-from medical device design and manufacturing to full-scale production.
Here are key benefits of working with a leading contract manufacturer:
- Turnkey Solutions: A manufacturing partner that offers design and engineering expertise along with validation services provides a complete solution. This eliminates the need for multiple vendors, saving you time and reducing complexity.
- Regulatory Expertise: Navigating FDA requirements is a critical part of the medical devices product development process. A strong development partner understands these regulations and incorporates them into the development and production phases, reducing the risk of delays.
- Consistency and Quality: With an experienced partner, your product benefits from established quality control processes. Whether you’re developing a new device or scaling an existing one, a reliable manufacturer will maintain high standards throughout production.
Overcoming Challenges in Medical Device Development
Bringing a medical device to the US market isn’t without its hurdles. Regulatory requirements, strict timelines, and the need for validation can complicate the process. However, partnering with a contract manufacturer in the USA that provides a diverse range of development services and validation solutions can help you tackle these challenges head-on.
Here are some common challenges and how the right partner can help:
- Regulatory Compliance: FDA regulations can be complex, but a manufacturer with experience in medical device design and manufacturing will have the knowledge to guide you through the approval process, making sure your product meets all necessary standards.
- Validation and Verification: Without proper manufacturing process validation, you could face delays or even rejection in the US market. A reliable manufacturer integrates validation from the start, reducing the risk of setbacks.
- Speed to Market: Tight deadlines are common in the medical device industry. A strong manufacturing partner helps you maintain momentum throughout the medical devices product development process by offering streamlined services, from prototyping to production.

Accelerate Your Medical Device's Path to Market with MFG One
Contact us today to learn more about how we can support your medical device design and manufacturing needs and help you achieve success in the US market and beyond.
