
Table of Contents
The Importance of User-Centered Design in Medical Device Engineering
Key benefits of user-centered design in medical device engineering include:
- Improved Usability: Devices that are simple to operate allow healthcare professionals to focus on patient care rather than figuring out how the device works.
- Reduced Errors: By designing devices that are easy to understand and operate, human factors engineering helps prevent mistakes that could compromise patient safety.
- Improved User Satisfaction: Medical equipment designed for comfort and efficiency improves the user experience, fostering trust and encouraging easy adoption.
How Human Factors Engineering Fits Into Medical Device Development
The process typically involves:
- User Research and Testing: Collecting data on how real users interact with the device to identify potential pain points.
- Prototype Development: Creating multiple iterations of the device to test and refine usability, ergonomics, and functionality.
- Design Adjustments: Implementing changes based on user feedback and testing outcomes to optimize the device for ease of use and performance.
Regulatory Requirements for Human Factors Engineering
Key regulatory considerations include:
- FDA Guidelines: The FDA mandates that human factors be evaluated to make sure medical equipment is safe and effective for their intended use. This includes thorough manufacturing process validation and usability testing.
- ISO 13485 Compliance: ISO standards require that human factors are integrated into the design process to minimize user error and improve device functionality.

Real-World Benefits of Applying Human Factors Engineering
Key benefits include:
- Reduced Product Recalls: By identifying potential user errors early in the product development process, manufacturers can minimize the chances of costly recalls.
- Improved Patient Safety: A user-centered design makes sure that healthcare professionals can operate medical equipment intuitively, minimizing the risk of human error.
- Increase Device Effectiveness: When human factors engineering is applied during device design and manufacturing, the result is a product that meets both user and regulatory expectations, delivering a reliable and effective solution to the market.
- Lower Training Costs: Intuitive design reduces the learning curve for healthcare professionals, decreasing the resources needed for training and customer support.
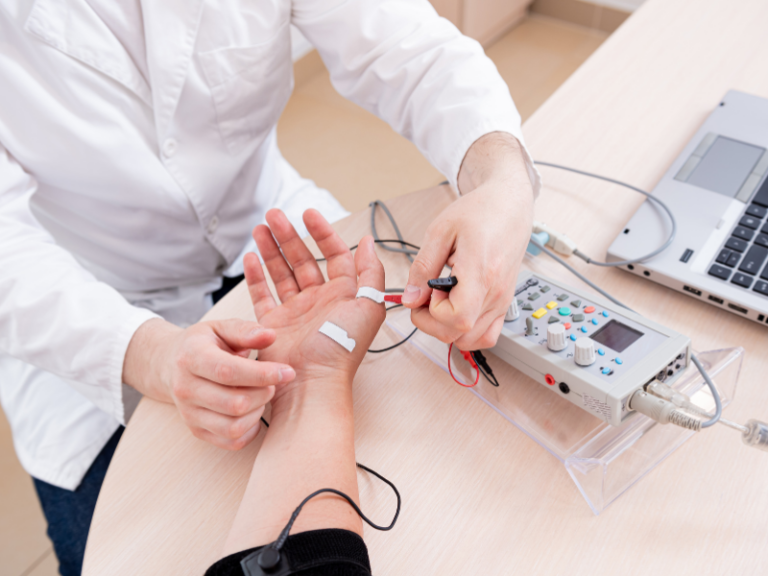
Collaboration Between Human Factors Engineers and Product Designers
In medical device engineering, seamless collaboration between human factors engineers and product designers is important. This partnership makes sure that devices are both functional and user-friendly, meeting the needs of healthcare professionals and patients alike.
The collaboration process typically includes:
- Early Integration of Human Factors: Human factors engineers work closely with product design teams right from the initial stages, offering insights on user behavior and interface design.
- Iterative Design Refinements: Together, they conduct multiple rounds of testing and prototyping to make sure that the device operates as intended, while also meeting usability standards.
- Feedback-Driven Adjustments: Product designers and human factors experts continuously exchange feedback, adjusting the design based on test results to maintain optimal user experience.

Partner with MFG One for User-Centered Medical Device Design
Incorporating human factors engineering into medical device engineering is important to developing products that are safe, effective, and user-friendly. Companies can reduce errors, improve usability, and ultimately deliver better outcomes by considering the needs of healthcare professionals and patients from the earliest stages of product development. Whether it’s aligning with registered medical device manufacturers or meeting ISO certification standards, focusing on human factors plays a critical role in the successful design and performance of medical equipment.
If you’re looking to develop your next medical device with a focus on human-centered design, MFG One is here to help. With expertise in medical device design and manufacturing, ISO-certified manufacturing, and more across the globe, including the US, Canada, the United Kingdom, and Mexico, we provide the comprehensive support you need to bring your product to market.
