This guide explores the factors fueling this growth, key market trends, and how established OEMs can leverage the right partnership with contract manufacturers to succeed.
Table of Contents
A Guide to the Medical Device Contract Manufacturing Market
Innovations in medical technology, from wearable sensors to complex diagnostic equipment, are driving growth in the medical device contract manufacturing market as companies seek out specialized expertise to bring their ideas to life.
This guide explores the factors fueling this growth, key market trends, and how established OEMs can leverage the right partnership with contract manufacturers to succeed.

Custom bio-medical devices produced in a controlled environment. Background shows a fraction of the floor space of a full-service contract manufacturing partner, providing a specialized, end-to-end integration of complex electronic subsystems.
Why Do Established OEMs Outsource Medical Device Design Manufacturing Services?
Partnering with a contract manufacturer is now a core part of the business strategy for most established medical device companies. The primary drivers for this shift are the increasing complexity of devices, which often require deep medical device engineering expertise and the stricter regulatory hurdles that demand a specific focus on quality and compliance.
By partnering with manufacturing experts, OEMs can focus their resources on their core competencies, like R&D, clinical trials, and sales, while leveraging the benefits of contract manufacturing to handle the capital-intensive production process.

A convective patient warming system, representing one of the Specialized Medical Equipment Builds that contract manufacturers produce. OEMs partner with manufacturers in the medical device contract manufacturing market to build, assemble, and test the core electromechanical systems for complex therapeutic devices.
How Big Is The Medical Device Contract Manufacturing Market?
The medical device contract manufacturing market, which is currently worth tens of billions of dollars, is projected to keep growing significantly. Precedence Research reports that the global market was worth $90.29 billion in 2024 and expects say it will reach $253.86 billion by 2034, with a compound annual growth rate (CAGR) of 10.89%.
This expansion is driven by a combination of factors, including rising global demand for healthcare, an aging population, and continuous technological advancements in medical technology. For OEMs, this data signifies a growing network of specialized partners ready to support their projects in the growing medical device contract manufacturing market.
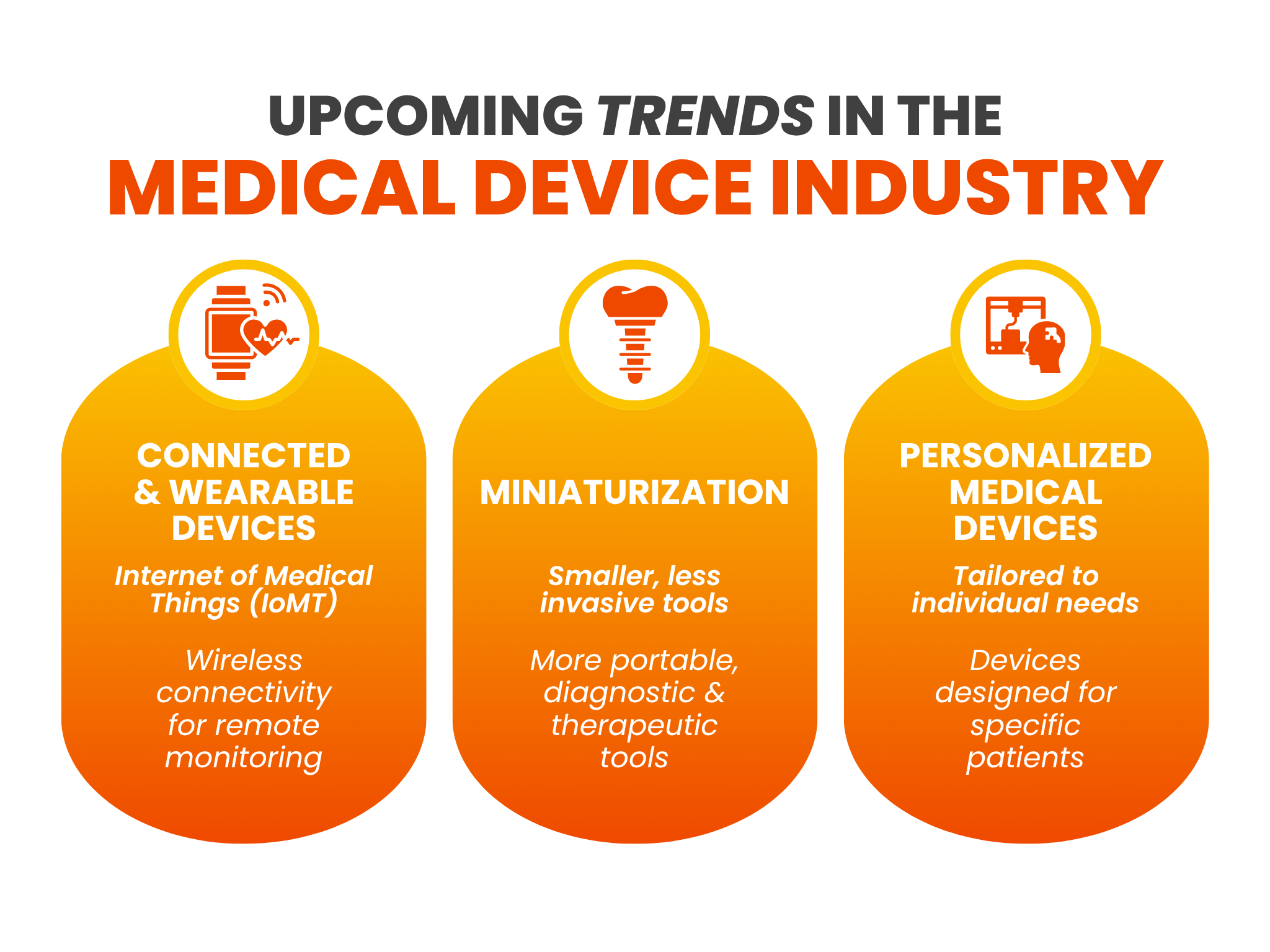
What are the upcoming trends in the medical device industry?
Several industry trends are shaping the future of healthcare and its industry growth. Key developments that both OEMs and their medical device manufacturing partners in the USA should consider include:
- Connected & Wearable Devices: The rise of the Internet of Medical Things (IoMT) is creating demand for devices with wireless connectivity for remote monitoring. An example is a wearable cardiac monitor that transmits real-time patient data directly to a healthcare provider’s dashboard. Producing this device requires expertise in both electronics miniaturization and data security.
- Miniaturization: There is a firm push toward smaller, less invasive, and more portable diagnostic and therapeutic tools in the medical device contract manufacturing market. Handheld ultrasound wands and micro-implants development are examples of this trend and require high-precision assembly and advanced materials.
- Personalized Medical Devices: The industry is moving away from one-size-fits-all products toward devices and instruments designed to individual patient needs. This requires flexible product design and development for manufacturing items like patient-specific 3D-printed surgical guides or custom-calibrated diagnostic instruments.
Key Medical Device Manufacturing Applications
A manufacturer’s true value in the medical device contract manufacturing market is its ability to handle a range of critical, high-complexity applications. Rather than just simple assembly, this includes manufacturing complex electromechanical subsystems that form the core of larger medical systems.
MFG One, for instance, is a medical device contract manufacturing partner that supports key market applications in the medical device contract manufacturing market, including:
- Fluid Warming Systems: We provide turnkey manufacturing and box-build assembly for a range of advanced fluid warming systems and rapid infusers for other medical companies.
- Convective Patient Warming Systems: We build and assemble the core components and electromechanical systems used in convective patient warming units.
- Medical Device Enclosures and Housings: Our medical device engineering expertise extends to the production of high-quality, durable, and compliant enclosures and housings for a variety of specialized devices.
- Portable and Wearable Medical Devices: We support the growing trend of portable and wearable technology, from product design and development support to final, scaled assembly.
- Specialized Medical Equipment Builds: Our capabilities cover a wide range of specialized, custom medical equipment builds and subsystems to meet unique OEM requirements.
How Can Established OEMs Leverage Manufacturing Partnerships?
Unlike startups, established OEMs face unique challenges of scale, legacy product support, and lifecycle management. For them, a strategic partnership with a medical device manufacturer is about optimization and risk management. It allows them to:
- Manage Fluctuating Demand: An OEM can scale production up or down to meet market demand without the massive capital investment of retooling or idling an in-house facility.
- Mitigate Regulatory & Supply Chain Risk: A dedicated partner with an established Quality Management System can manage the complexities of global supply chains and evolving regulatory demands, freeing the OEM from this significant burden.
- Focus Internal Resources: By outsourcing production, an OEM’s internal engineering team can be re-allocated from managing legacy production lines to focusing on next-generation R&D and innovation.
These industrial manufacturing services allow an OEM to effectively leverage the specialized expertise of the broader medical device contract manufacturing market as a direct extension of their own capabilities.
What to Look for in a Medical Device Manufacturer
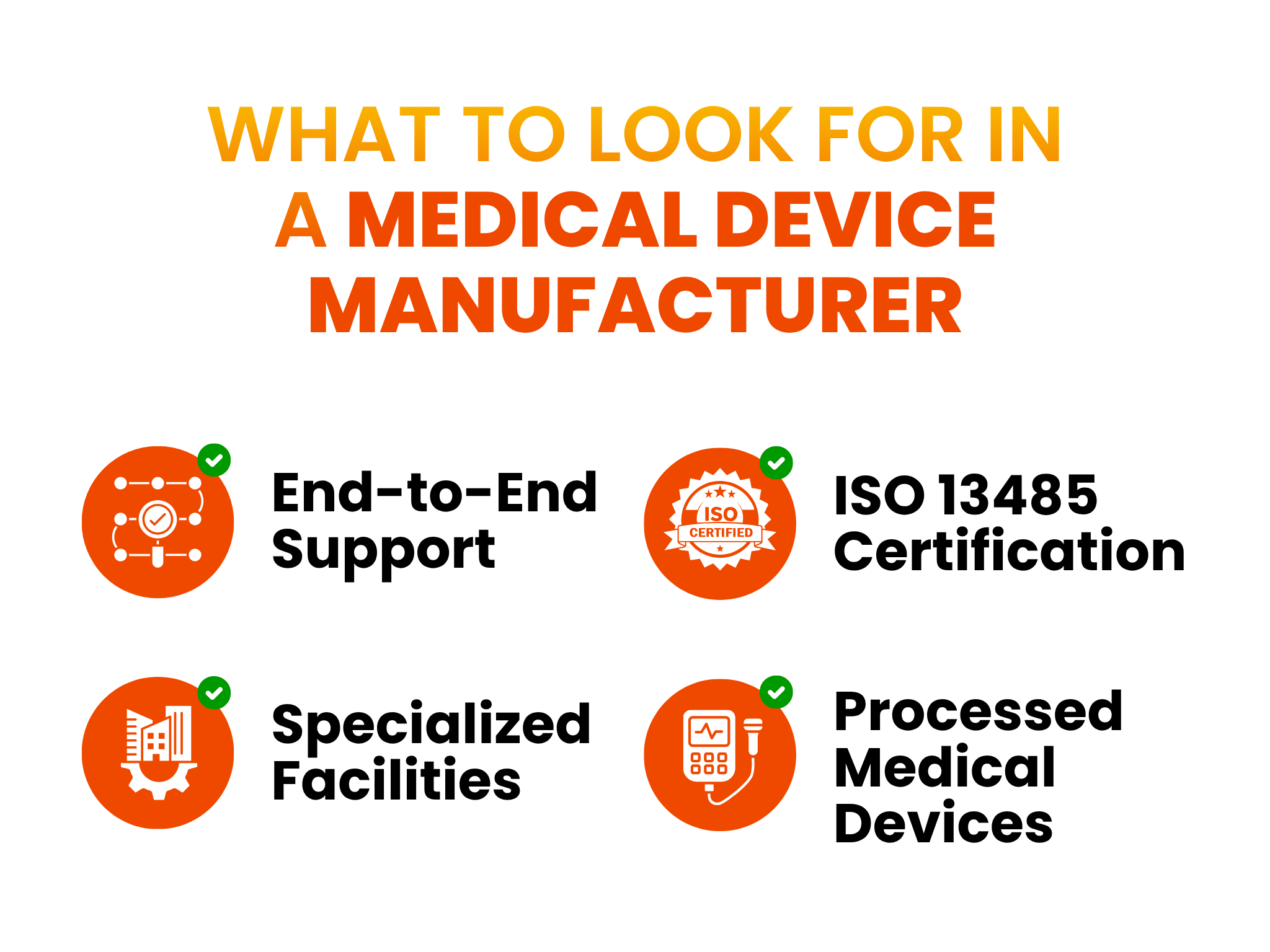
Navigating these trends in medical device production requires a partner that handles all manufacturing processes under one roof. Today’s leading partners are full-service providers offering end-to-end support, from initial medical device design manufacturing services to final packaging and lifecycle management.
They operate specialized, highly controlled facilities and hold the critical certifications that are non-negotiable in this industry. Chief among these is the ISO 13485 contract manufacturer certification, which shows a proven commitment to the quality and regulatory demands of the medical device industry.
Your Partner in a Growing Medical Device Market
The growing medical device contract manufacturing market presents a powerful opportunity for any medical device company. Success hinges on choosing a partner who offers not just production capacity, but integrated, end-to-end services with a proven quality system.
As a leading box build electronic contract manufacturer, MFG One provides this kind of support. Our experienced teams serve clients across the United States, Canada, the United Kingdom, Mexico and beyond.
