Table of Contents
Understanding Regulatory Requirements
Common Challenges in Medical Device Validation
- Time-Consuming Processes: Validation often involves multiple rounds of testing, documentation, and approvals. For FDA-registered device manufacturers, this can lead to lengthy delays if not properly managed.
- High Costs: The cost of validation, from testing equipment to regulatory fees, can quickly add up. Partnering with a trusted contract manufacturer that offers comprehensive validation services can help manage these costs through efficient testing and documentation strategies.
- Navigating Changing Regulations: Regulatory standards for medical devices frequently change, and keeping up with these updates can be difficult. A contract manufacturer that’s experienced in medical device new product development and familiar with current standards can help you stay compliant while avoiding unnecessary delays.
3 Key Steps for Effective Medical Device Validation
Developing a solid validation strategy is important when it comes to reducing delays and avoiding unnecessary costs during the medical device new product development phase. By planning ahead and integrating validation into each phase of your process, you can maintain smoother regulatory approval and faster time-to-market.
Here are the key three steps for effective validation:
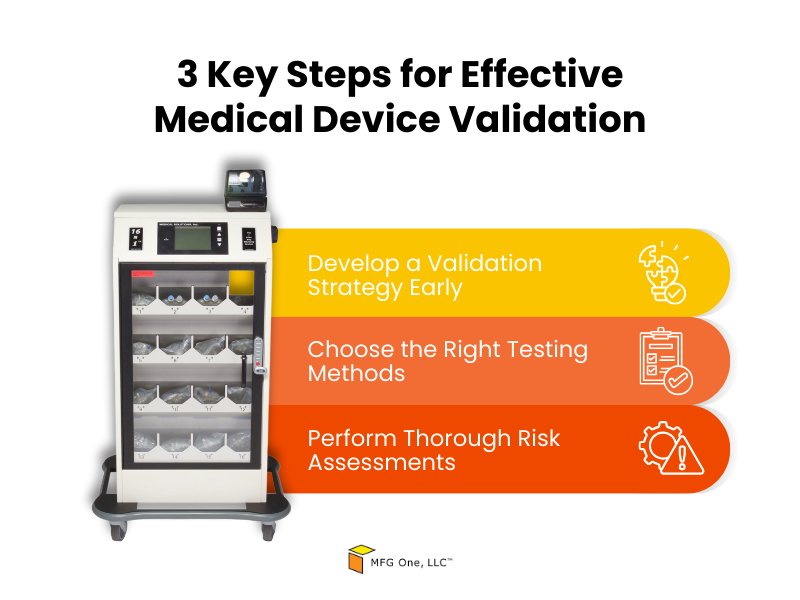
- Develop a Validation Strategy Early: Building a clear validation plan from the start helps you anticipate regulatory requirements and testing needs. Involving product development services early on will allow you to identify potential validation challenges before they escalate.
- Choose the Right Testing Methods: Selecting appropriate testing methods is essential for demonstrating that your product meets regulatory standards. A good partner will help you choose the right tools and testing environments for medical device new product development and validation to avoid unnecessary delays.
- Perform Thorough Risk Assessments: Assessing potential risks early helps you address them proactively. This involves evaluating product design, manufacturing processes, and any regulatory challenges that may arise.
The Role of Contract Manufacturers and External Partners
Partnering with the right medical contract manufacturer can simplify the often overwhelming validation process. A contract manufacturing company with expertise in medical device new product development not only helps you meet regulatory requirements but also makes sure that validation is seamlessly integrated into every phase of product development.
Here’s how external partners can help:
- Expertise in Validation Requirements: Experienced manufacturers understand the specific validation needs for medical devices, including process validation and risk assessments, helping you avoid costly errors and delays.
- Streamlined Validation Process: A strong partner can assist in managing the documentation and testing required for validation. This simplifies your workflow and saves valuable time.
- Holistic Approach: With services that include everything from product development solutions to EMS contract manufacturing, the right partner offers a full range of solutions, reducing the need for multiple vendors and making the validation process more efficient.

Best Practices to Streamline the Validation Process
For a smoother medical device new product development journey, integrating validation early and staying proactive is key. By following best practices, you can avoid delays and keep your project on track, especially when working with regulatory bodies like the FDA.
Here are some best practices to consider:
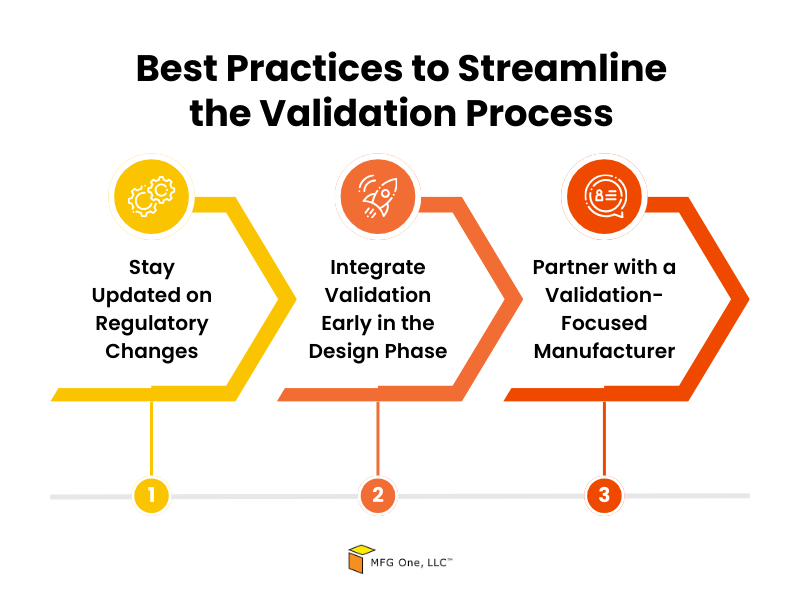
- Stay Updated on Regulatory Changes: Regulations for medical devices frequently evolve. Keep yourself informed on updates from the FDA and other regulatory bodies to avoid compliance issues.
- Integrate Validation Early in the Design Phase: Incorporating validation into the initial stages of medical device design and manufacturing helps identify and address potential issues before they become costly obstacles. It also makes the overall validation process smoother and faster.
- Partner with a Validation-Focused Manufacturer: Working with a contract manufacturer that prioritizes validation from the start makes sure that your product is designed with compliance in mind. This can drastically reduce the time it takes to get your product to market.
Streamline Your Medical Device Validation New Product Development Process with the Right Partner
Bringing a medical device to market doesn’t have to be overwhelming. With the right partner, you can streamline medical device new product development with confidence, speed, and precision. At MFG One, we’re more than just a manufacturer-we’re your strategic ally in making sure every stage, from product development to process validation, is streamlined and efficient. Our expertise in regulatory compliance helps you avoid delays and get your product to market faster.
Contact us today to learn how we can support your medical device new product development efforts and simplify your validation process.
